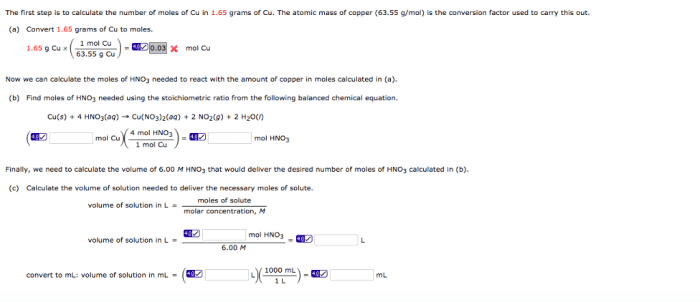
How many moles are in 199 grams of CCl4? This question delves into the fundamental concepts of molecular mass, molar mass, and their significance in chemistry. Understanding this conversion is crucial for various chemical calculations and applications.
To determine the number of moles in 199 grams of CCl4, we must first establish its molecular mass and then employ the formula that relates mass, molecular mass, and moles.
Molecular Mass and Mole Concept: How Many Moles Are In 199 Grams Of Ccl4
The molecular mass of a compound is the sum of the atomic masses of all the atoms in its molecule. It is expressed in atomic mass units (amu). The mole is the SI unit of amount of substance, defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
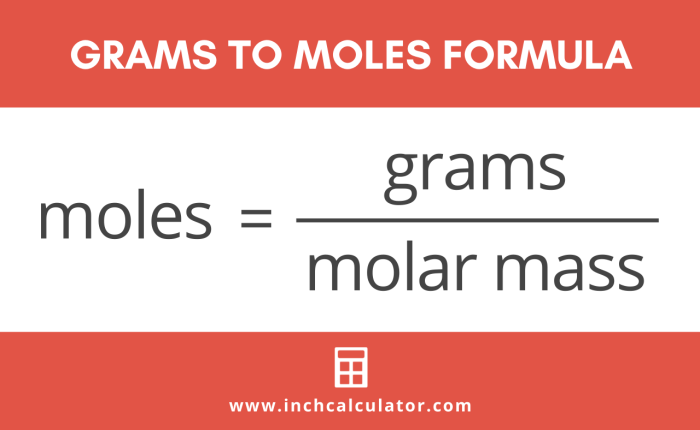
The number of moles of a substance is calculated by dividing its mass by its molecular mass.
Determining the Molecular Mass of CCl4
CCl4 is composed of one carbon atom, four chlorine atoms, and has a molecular mass of 153.82 amu. This is calculated by adding the atomic masses of one carbon atom (12.01 amu) and four chlorine atoms (35.45 amu each).
Converting Grams to Moles, How many moles are in 199 grams of ccl4
To convert grams to moles, we use the following formula:
Number of moles = Mass (in grams) / Molecular mass (in amu)
Interpreting the Result
In the case of 199 grams of CCl4, the number of moles is:
Number of moles = 199 grams / 153.82 amu = 1.29 moles
This information can be used in further chemical calculations, such as determining the number of molecules, atoms, or ions in the sample.
Detailed FAQs
What is the molecular mass of CCl4?
The molecular mass of CCl4 is 153.82 g/mol.
How many moles are in 199 grams of CCl4?
To calculate the number of moles, we divide the mass by the molecular mass: 199 g / 153.82 g/mol = 1.29 moles.
What is the significance of converting grams to moles?
Converting grams to moles allows us to determine the number of molecules or atoms present, calculate reaction stoichiometry, and predict the behavior of substances in chemical reactions.